Bioavailability Study Against
Aerosol Spray
Bioavailability Study Against Aerosol Spray
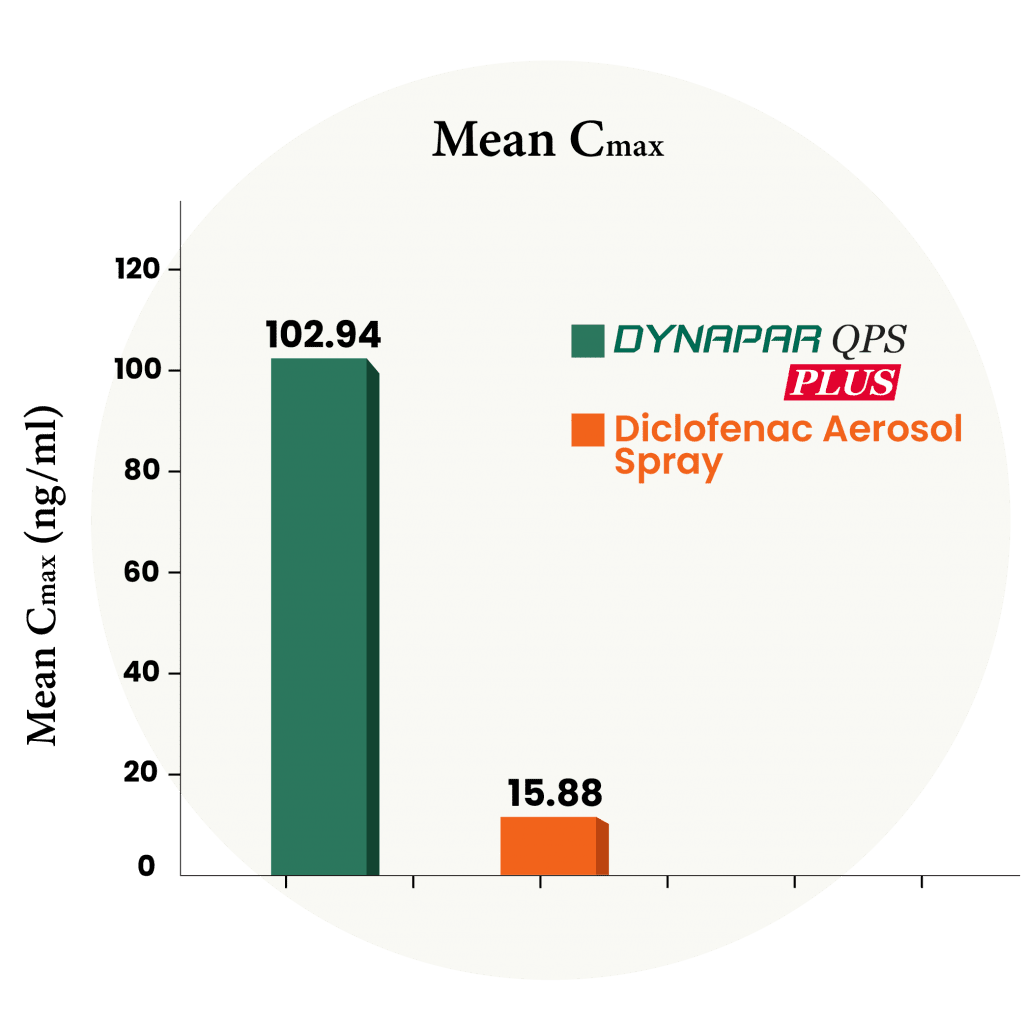
6X Higher absorption compared to Diclofenac Aerosol Spray
In this study, a group of people received Dynapar QPS Plus or marketed Diclofenac aerosol spray. Blood samples were taken before the dose and up to 24 hours after. The levels of Diclofenac were measured using the latest bioanalytical method. Dynapar QPS Plus significantly enhances the delivery of medication into the skin, outperforming traditional diclofenac aerosol sprays by six times in absorption. Dynapar QPS Plus ensures precise dosing with a metered dose spray pump. No adverse event occurred during this study.
Dynapar QPS Plus delivers 6X higher absorption than aerosol sprays, ensuring precise dosing, superior efficacy, and a safe user experience.
Bioavailability Study Against
Diclofenac Gel
Bioavailability Study Against Diclofenac Gel
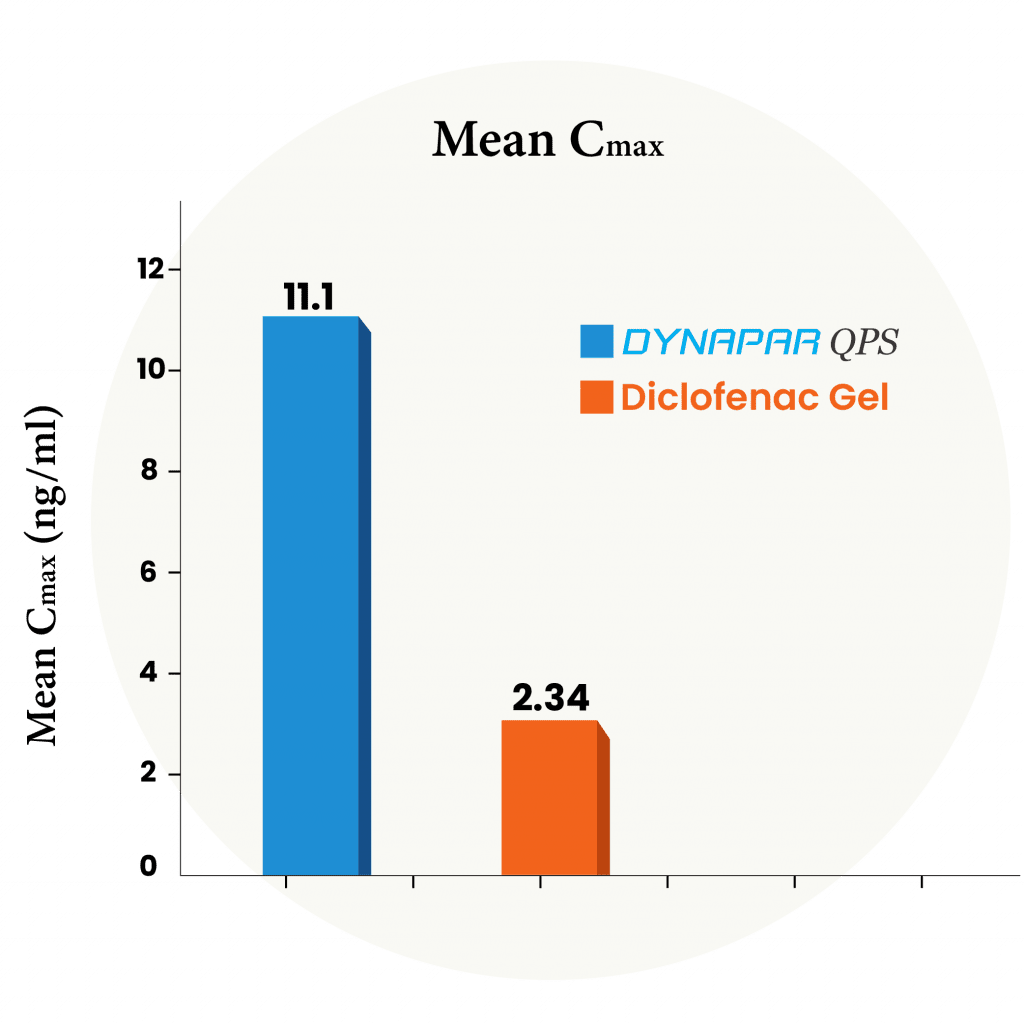
10X Higher absorption compared to Diclofenac Gel
In this study, a group of people received Dynapar QPS or marketed Diclofenac gel. Blood samples were taken before the dose and up to 24 hours after. The levels of Diclofenac were measured using the latest bioanalytical method. This study confirmed that absorption of diclofenac from topical administration of Dynapar QPS is faster and approximately 10 times higher than that of diclofenac gels and thus provides faster and higher concentration in the underlying tissues of the skin and provides faster and better efficacy.
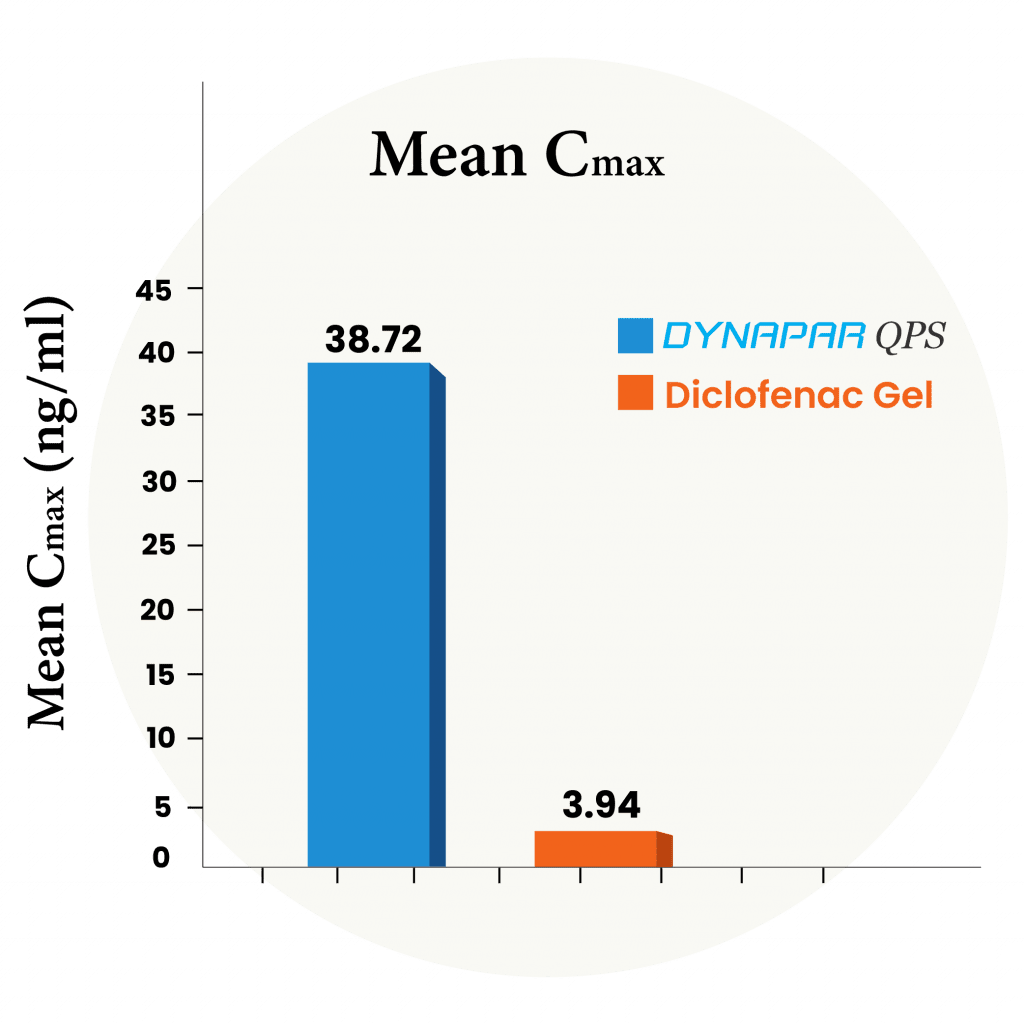
*Data on file
Dynapar QPS offers 10X higher Diclofenac absorption than diclofenac gels, enabling faster action, deeper penetration and superior pain relief effectiveness
Dermal Microdialysis Study
Against Diclofenac Gel
Dermal Microdialysis Study Against Diclofenac Gel
5X penetration at the site of pain
Dermal Microdialysis was conducted to determine how much Diclofenac enters the skin after applying Dynapar QPS compared to a Conventional Topical Pain Reliever. In this study, a special tube (Microdialysis catheter) was placed beneath the skin, which allows substances to pass through. The Topical pain reliever and Dynapar QPS were applied on marked patches of skin, one after the other. A small pump circulated a fluid through the tube, and any diclofenac that crossed the skin entered the tube and mixed with the fluid. The samples of this fluid were taken at regular intervals to measure the amount of diclofenac that crossed the skin. The study established that more diclofenac entered the fluid in the case of Dynapar QPS compared to the Topical Pain Reliever. Dynapar QPS a targeted drug delivery method, ensuring a higher concentration of the medication reaches the stratum corneum and skin specifically at the site of pain. This study demonstrated Dynapar QPS demonstrates 5 times higher penetration at the site of pain, thereby effectively reducing the necessity for oral painkillers and reducing the potential risk of severe organ failure associated with oral painkillers.
Dynapar QPS delivers 5X higher skin penetration of diclofenac, enabling targeted pain relief while minimizing oral painkiller side effects.
Clinical Trial
Clinical Trial
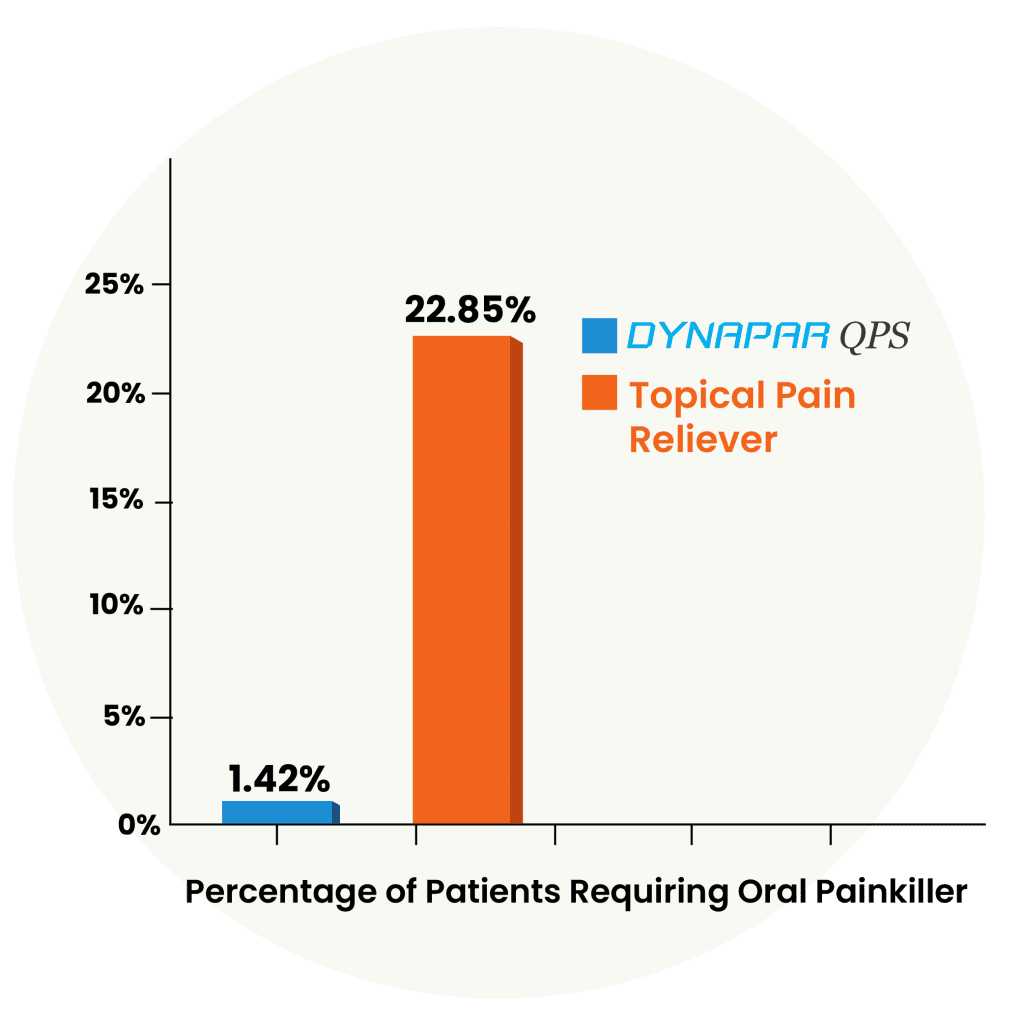
The reduction in consumption of oral painkillers in patients on Dynapar QPS is due to its 5X Penetration.
A clinical trial conducted by independent investigators has established that the pain relief provided by Dynapar QPS is so powerful that patients consumed lesser oral painkillers. This is due to its 5X penetration and powerful absorption efficacy at the site of pain, which reduces the need for oral painkillers,reduces the risk of acute kidney failure and heart-related diseases. In contrast, higher 22.85% of patients who used a Topical Pain Reliever still needed to take oral painkillers as well.
Dynapar QPS significantly reduces the requirement of oral painkillers, thereby minimizing risks to the kidneys and heart.
Scintigraphy Study
Scintigraphy Study
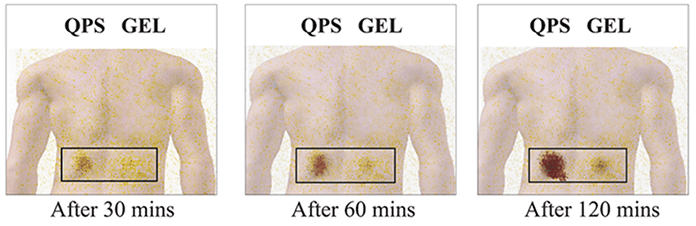
This study was conducted by the Department of Nuclear Medicine, Institute of Nuclear Medicine and Allied Sciences (lNMAS), Delhi. In this study, ‘Diclofenac’ was tagged with radioactive material (Technetium) in Dynapar QPS as well as the reference Topical Pain Reliever. Both the formulations were then applied on the knees and backs of the volunteers. The radioactivity levels were thereafter measured at regular intervals for both Dynapar QPS as well as the Reference Topical Pain Reliever.
In case of Dynapar QPS, the radioactivity was significantly higher compared to Topical Pain Reliever, indicating higher penetration of diclofenac for Dynapar QPS vis-a-vis the reference Topical Pain Reliever.
The study confirms significantly higher skin penetration of diclofenac from Dynapar QPS compared to the reference Topical Pain Reliever
Franz Diffusion Study
Franz Diffusion Study
The Franz diffusion study evaluated the ability of Dynapar QPS and other Topical Pain Relievers to penetrate the skin. It used a synthetic Strat-M Membrane, which mimics human skin, to test the diffusion of the drugs. The study found that Dynapar QPS had three times higher penetration of diclofenac compared to other globally available topical pain relievers. This study confirms that Dynapar QPS has the highest transdermal penetration* among all topical pain relievers, making it the best solution.
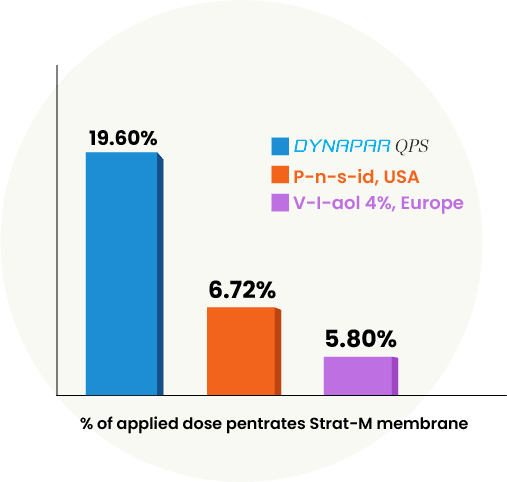
*Data on file
Dynapar QPS shows 3X higher transdermal penetration, proving to be the most effective topical solution for lasting pain relief.